COMPANY NEWS
Essex Bio-Technology and Mitotech announce positive outcome of VISTA-2 Phase 3 clinical study in Dry Eye Disease
2021.02.24
Download
Both VISTA-1 and VISTA-2 demonstrated significant impact of SkQ1 on clearing of Central Fluorescein Staining and improvement of Best Corrected Visual Acuity (BCVA) at day 28 (p<0.05 for both endpoints in VISTA-1 and VISTA-2), providing clear guidance for primary endpoint designation in the next pivotal study for the purpose of NDA submission.
Hong Kong, 24 Feb 2021
Essex Bio-Technology Ltd (“EssexBio” or the “Group”, Stock Code: 1061.HK) and Mitotech S.A. (“Mitotech”), a Luxembourg-based clinical-stage biotechnology company, announced positive data from their VISTA-2 study (NCT04206020) – a U.S. randomized, double-blind, vehicle-controlled study of SkQ1 as treatment for Dry Eye Disease (DED).
VISTA-2 was a multi-centre, randomized, double-blind, placebo-controlled clinical study with two treatment arms: BID SkQ1 ophthalmic solution and BID vehicle. 610 patients were enrolled in the study across more than a dozen multiple centres in the U.S. and received treatment over a 2-month period.
"VISTA-2 results enable a unique and clear plan for our next study VISTA-3", said Natalia Perekhvatova, Chief Executive Officer of Mitotech S.A. "Consistent efficacy signal in our predetermined key secondary sign metric - central corneal staining at day 28 – that we see across both VISTA-1 and VISTA-2 datasets is very encouraging. We are very optimistic ahead of our meeting with the U.S. FDA this Spring, where we will be looking to confirm with the agency Mitotech’s next development steps targeting NDA submission in 2022-2023."
One of study’s predetermined key secondary endpoints – change from baseline in central fluorescein staining after just 28 days of treatment – demonstrated statistically significant signal (p<0.05) against vehicle group in a subgroup of patients characterized by higher Schirmer’s scores. Importantly, this efficacy signal was observed in both VISTA-1 and VISTA-2 studies for that subgroup of patients, driving p-value for the combined two-study dataset to <0.0005. Same subgroup of patients demonstrated statistically significant clearing of central fluorescein staining (defined as zero staining in central cornea) and improvement of best corrected visual acuity (BCVA) at day 28 in both studies (p<0.05 for both endpoints in VISTA-1 and VISTA-2).
Consistent signal in these secondary endpoints provides clear guidance for primary endpoint designation in Mitotech’s next pivotal study for the purpose of NDA submission, replacing VISTA-2 nominal co-primaries (conjunctival fluorescein staining and ocular discomfort at day 56).
This VISTA-2 study continues to highlight excellent safety profile of the drug with tolerability being statistically similar to that of an artificial tear.
"Both VISTA-1 and VISTA-2 outcomes point to drug’s highly significant impact on central staining, and this is further supported by improvement of visual function – something that matters to patients", said George Ousler, Vice President, Dry Eye at Ora, Inc. "Statistically significant clearing of central staining against such comparator as artificial tear is a unique read-out. If confirmed in the next study, such result may have a very positive impact on Mitotech’s time to market – subject to company’s consultations with the FDA."
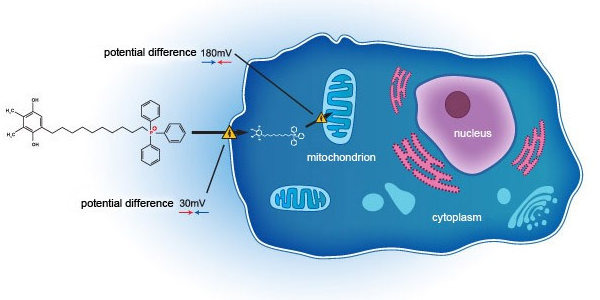
"SkQ1's mechanism of action against oxidative stress in the mitochondria is well displayed as we see efficacy in DED patient population (with excellent safety and tolerability profile) within VISTA-1 and VISTA-2", said Malcolm Ngiam, Deputy Managing Director of Essex Bio-Technology Limited. "We are excited with the read-out (clearing of central staining of the cornea), revealing the potential of SkQ1 in addressing oxidative stress in DED. Thus, we look forward to the impact of VISTA-3 on the overall regulatory pathway towards NDA."
Hong Kong, 24 Feb 2021
Essex Bio-Technology Ltd (“EssexBio” or the “Group”, Stock Code: 1061.HK) and Mitotech S.A. (“Mitotech”), a Luxembourg-based clinical-stage biotechnology company, announced positive data from their VISTA-2 study (NCT04206020) – a U.S. randomized, double-blind, vehicle-controlled study of SkQ1 as treatment for Dry Eye Disease (DED).
VISTA-2 was a multi-centre, randomized, double-blind, placebo-controlled clinical study with two treatment arms: BID SkQ1 ophthalmic solution and BID vehicle. 610 patients were enrolled in the study across more than a dozen multiple centres in the U.S. and received treatment over a 2-month period.
"VISTA-2 results enable a unique and clear plan for our next study VISTA-3", said Natalia Perekhvatova, Chief Executive Officer of Mitotech S.A. "Consistent efficacy signal in our predetermined key secondary sign metric - central corneal staining at day 28 – that we see across both VISTA-1 and VISTA-2 datasets is very encouraging. We are very optimistic ahead of our meeting with the U.S. FDA this Spring, where we will be looking to confirm with the agency Mitotech’s next development steps targeting NDA submission in 2022-2023."
One of study’s predetermined key secondary endpoints – change from baseline in central fluorescein staining after just 28 days of treatment – demonstrated statistically significant signal (p<0.05) against vehicle group in a subgroup of patients characterized by higher Schirmer’s scores. Importantly, this efficacy signal was observed in both VISTA-1 and VISTA-2 studies for that subgroup of patients, driving p-value for the combined two-study dataset to <0.0005. Same subgroup of patients demonstrated statistically significant clearing of central fluorescein staining (defined as zero staining in central cornea) and improvement of best corrected visual acuity (BCVA) at day 28 in both studies (p<0.05 for both endpoints in VISTA-1 and VISTA-2).
Consistent signal in these secondary endpoints provides clear guidance for primary endpoint designation in Mitotech’s next pivotal study for the purpose of NDA submission, replacing VISTA-2 nominal co-primaries (conjunctival fluorescein staining and ocular discomfort at day 56).
This VISTA-2 study continues to highlight excellent safety profile of the drug with tolerability being statistically similar to that of an artificial tear.
"Both VISTA-1 and VISTA-2 outcomes point to drug’s highly significant impact on central staining, and this is further supported by improvement of visual function – something that matters to patients", said George Ousler, Vice President, Dry Eye at Ora, Inc. "Statistically significant clearing of central staining against such comparator as artificial tear is a unique read-out. If confirmed in the next study, such result may have a very positive impact on Mitotech’s time to market – subject to company’s consultations with the FDA."
"SkQ1's mechanism of action against oxidative stress in the mitochondria is well displayed as we see efficacy in DED patient population (with excellent safety and tolerability profile) within VISTA-1 and VISTA-2", said Malcolm Ngiam, Deputy Managing Director of Essex Bio-Technology Limited. "We are excited with the read-out (clearing of central staining of the cornea), revealing the potential of SkQ1 in addressing oxidative stress in DED. Thus, we look forward to the impact of VISTA-3 on the overall regulatory pathway towards NDA."
~ End ~
About Essex
Essex Bio-Technology Limited is a bio-pharmaceutical company that develops, manufactures and commercialises genetically engineered therapeutic rb-bFGF (FGF-2), having six commercialised biologics marketed in China since 1998. The products of the Company are principally prescribed for the treatment of wounds healing and diseases in Ophthalmology and Dermatology, which are marketed and sold through approximately 9,000 hospitals and managed directly by its 43 regional sales offices in China. Leveraging on its in-house R&D platform in growth factor and antibody, the Company maintains a pipeline of projects in various clinical stages, covering a wide range of fields and indications.
About Mitotech S.A.
Mitotech S.A. is a Luxembourg-based biotechnology company developing novel drugs for treatment of mitochondrial oxidative stress in predominantly age-related disorders. The core technology behind Mitotech products is based on a novel class of small molecules – mitochondria targeting cardiolipin peroxidation inhibitors. VISTA-1 – a Phase 2b/3 clinical study in the United States (NCT03764735) - SkQ1 showed evidence of efficacy in reducing both the signs and symptoms in DED patients. Dry AMD and LHON programs for SkQ1 ophthalmic solution are at pre-Phase 2 stage with Phase 2 studies projected to start in 2021.
About Ora®, Inc.
Ora is the world's leading full-service ophthalmic research organization with offices in the United States, Europe, Australia, and Asia. For over 40 years, we have helped our clients earn more than 45 product approvals. We support a wide array of organizations, from start-ups to global pharmaceutical and device companies, to efficiently bring their new products from concept to market. Ora's unique models, methodologies, and global regulatory strategies have been refined and proven across thousands of global projects. We bring together the world's most extensive and experienced team of ophthalmic experts to maximize the value of new product initiatives. For more information, please visit www.oraclinical.com.
About George Ousler
George Ousler has 20 years of pioneering pharmaceutical development in the area of DED. He has authored over 200 publications on dry eye and has been invited to present his research at numerous national and international symposia. He serves on several noted DED research committees. As Vice President of Dry Eye Department at Ora, Inc (a world leading DED-centric CRO), George develops clinical models and regulatory pathways for the evaluation of dry eye therapies and has studied many of the agents under investigation.






















































 粵公網安備 44049102496184號
粵公網安備 44049102496184號