COMPANY NEWS
Essex Bio-Technology and Mitotech Announce First Patient First Visit in U.S. FDA Second Phase 3 Clinical Trial of SkQ1 ?
2019.12.12
Download
Hong Kong, 12 December 2019
Essex Bio-Technology Limited (“EssexBio” or the “Group”, Stock Code: 1061) is pleased to announce that a project invested by Essex Bio-Investment Limited, a wholly-owned subsidiary of the Group, in relation to a clinical development in a U.S. FDA second Phase 3 clinical trial of SkQ1 compound in patients with moderate to severe Dry Eye Disease (“DED”) has achieved pivotal progress.
A Phase 3 clinical study in DED patients has been initiated by EssexBio and Mitotech S.A. (“Mitotech”), targeting confirmation of the early onset of improvements across Dry Eye signs and symptoms demonstrated for SkQ1 ophthalmic solution in VISTA-1 study earlier this year.
EssexBio and Mitotech jointly announced of first patients in a Phase 3 study VISTA-2 building on positive results of VISTA-1 clinical study (a U.S. Phase 2b/3 study) of SkQ1 compound. SkQ1 belongs to the class of cardiolipin peroxidation inhibitors that is developing for treatment of a spectrum of age-related disorders, including DED. EssexBio has agreed to fund up to a maximum of US$$20,000,000 (equivalent to approximately HK$$156,600,000) for the second Phase 3 clinical trial.
“In a rare case for a Dry Eye Disease study data that came out of VISTA-1 delivered a clear message,” said Natalia Perekhvatova, Chief Executive Officer of Mitotech S.A.,“VISTA-1 results revealed early onset of action of SkQ1 for a spectrum of clinically relevant symptoms and signs such as Ocular Discomfort and Fluorescein Staining. Combined with drug tolerability comparable to that of an artificial tear this positions SkQ1 as an important potential treatment option for Dry Eye Disease patients worldwide.”
VISTA-1 was a multi-centre, randomized, double-blind, placebo-controlled clinical study involving three treatment arms: two concentrations of SkQ1 and vehicle, administered BID. Approximately 450 patients were enrolled in the study across multiple centres in the U.S. and receive treatment over a 2-month period. Nominal co-primary endpoints of the study (fluorescein staining in central corneal zone and grittiness symptom) were not met, but multiple predetermined secondary endpoints demonstrated broad action of SkQ1 in the intent to treat (ITT) population. Relative to the vehicle (an artificial tear) SkQ1 demonstrated statistically significant reduction of Ocular Discomfort (p<0.05) as early as after 4 weeks of treatment with multiple symptoms in 4-Symptom Questionnaire also demonstrating statistically significant reduction (p<0.05), all in ITT population. A global clinical sign - conjunctival fluorescein staining - demonstrated statistically significant reduction vs. vehicle (p<0.05), also in ITT population. At the same time the study highlighted excellent safety profile of the drug with tolerability being statistically similar to that of an artificial tear.
“We are very encouraged by the positive outcome in VISTA-1, supporting our decision to proceed with VISTA-2,” said Malcolm Ngiam, President of Essex Bio-Investment Limited, “We are excited to be able to continue our collaboration with Mitotech S.A. and to move one step closer to making SkQ1 available for the world-wide DED market.”
VISTA-2 is a multi-centre, randomized, double-blind, placebo-controlled clinical study, similar in its design to VISTA-1, but involving two treatment arms (SkQ1 solution and vehicle) and enrolling twice as many (300) patients per arm. VISTA-2 is currently expected to be completed by or around the second quarter of 2020.
For more information about VISTA-2 clinical trial will be available at www.clinicaltrials.gov soon.
About SkQ1
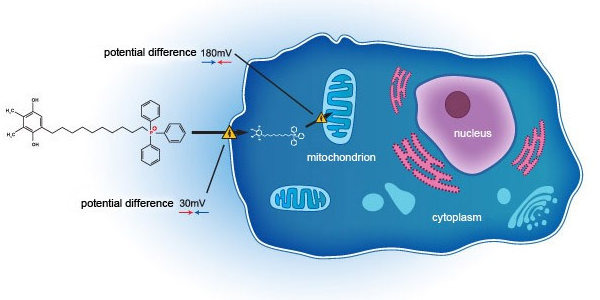
SkQ1 addresses DED through a novel mechanism of action, acting on the mitochondria on a cellular level. Unlike current standard of care, which acts primarily as anti-inflammatory agents, SkQ1 has been shown to not only relieve inflammation but also improve tissue degeneration and tear quality deficit by targeting oxidative stress within the eye. In VISTA-1 – a Phase 2b/3 clinical study in the United States (NCT03764735) - SkQ1 showed evidence of efficacy in reducing both the signs and symptoms in dry eye subjects.
About Mitotech S.A.
Mitotech S.A. is a Luxembourg-based biotechnology company developing novel drugs for treatment of predominantly age-related disorders. The core technology behind Mitotech products is based on a novel class of small molecules – mitochondria targeting cardiolipin peroxidation inhibitors. Company’s lead compound SkQ1 is being developed in several drug formulations covering a variety of therapeutic areas with major focus on ophthalmology and neurodegenerative diseases.
Essex Bio-Technology Limited (“EssexBio” or the “Group”, Stock Code: 1061) is pleased to announce that a project invested by Essex Bio-Investment Limited, a wholly-owned subsidiary of the Group, in relation to a clinical development in a U.S. FDA second Phase 3 clinical trial of SkQ1 compound in patients with moderate to severe Dry Eye Disease (“DED”) has achieved pivotal progress.
A Phase 3 clinical study in DED patients has been initiated by EssexBio and Mitotech S.A. (“Mitotech”), targeting confirmation of the early onset of improvements across Dry Eye signs and symptoms demonstrated for SkQ1 ophthalmic solution in VISTA-1 study earlier this year.
EssexBio and Mitotech jointly announced of first patients in a Phase 3 study VISTA-2 building on positive results of VISTA-1 clinical study (a U.S. Phase 2b/3 study) of SkQ1 compound. SkQ1 belongs to the class of cardiolipin peroxidation inhibitors that is developing for treatment of a spectrum of age-related disorders, including DED. EssexBio has agreed to fund up to a maximum of US$$20,000,000 (equivalent to approximately HK$$156,600,000) for the second Phase 3 clinical trial.
“In a rare case for a Dry Eye Disease study data that came out of VISTA-1 delivered a clear message,” said Natalia Perekhvatova, Chief Executive Officer of Mitotech S.A.,“VISTA-1 results revealed early onset of action of SkQ1 for a spectrum of clinically relevant symptoms and signs such as Ocular Discomfort and Fluorescein Staining. Combined with drug tolerability comparable to that of an artificial tear this positions SkQ1 as an important potential treatment option for Dry Eye Disease patients worldwide.”
VISTA-1 was a multi-centre, randomized, double-blind, placebo-controlled clinical study involving three treatment arms: two concentrations of SkQ1 and vehicle, administered BID. Approximately 450 patients were enrolled in the study across multiple centres in the U.S. and receive treatment over a 2-month period. Nominal co-primary endpoints of the study (fluorescein staining in central corneal zone and grittiness symptom) were not met, but multiple predetermined secondary endpoints demonstrated broad action of SkQ1 in the intent to treat (ITT) population. Relative to the vehicle (an artificial tear) SkQ1 demonstrated statistically significant reduction of Ocular Discomfort (p<0.05) as early as after 4 weeks of treatment with multiple symptoms in 4-Symptom Questionnaire also demonstrating statistically significant reduction (p<0.05), all in ITT population. A global clinical sign - conjunctival fluorescein staining - demonstrated statistically significant reduction vs. vehicle (p<0.05), also in ITT population. At the same time the study highlighted excellent safety profile of the drug with tolerability being statistically similar to that of an artificial tear.
“We are very encouraged by the positive outcome in VISTA-1, supporting our decision to proceed with VISTA-2,” said Malcolm Ngiam, President of Essex Bio-Investment Limited, “We are excited to be able to continue our collaboration with Mitotech S.A. and to move one step closer to making SkQ1 available for the world-wide DED market.”
VISTA-2 is a multi-centre, randomized, double-blind, placebo-controlled clinical study, similar in its design to VISTA-1, but involving two treatment arms (SkQ1 solution and vehicle) and enrolling twice as many (300) patients per arm. VISTA-2 is currently expected to be completed by or around the second quarter of 2020.
For more information about VISTA-2 clinical trial will be available at www.clinicaltrials.gov soon.
About SkQ1
SkQ1 addresses DED through a novel mechanism of action, acting on the mitochondria on a cellular level. Unlike current standard of care, which acts primarily as anti-inflammatory agents, SkQ1 has been shown to not only relieve inflammation but also improve tissue degeneration and tear quality deficit by targeting oxidative stress within the eye. In VISTA-1 – a Phase 2b/3 clinical study in the United States (NCT03764735) - SkQ1 showed evidence of efficacy in reducing both the signs and symptoms in dry eye subjects.
About Mitotech S.A.
Mitotech S.A. is a Luxembourg-based biotechnology company developing novel drugs for treatment of predominantly age-related disorders. The core technology behind Mitotech products is based on a novel class of small molecules – mitochondria targeting cardiolipin peroxidation inhibitors. Company’s lead compound SkQ1 is being developed in several drug formulations covering a variety of therapeutic areas with major focus on ophthalmology and neurodegenerative diseases.






















































 粵公網安備 44049102496184號
粵公網安備 44049102496184號